Patient-centred care:
turn the slogan into substance.
Health policies and programs are strongest when they are co-designed with the people they aim to benefit.
Real and constant engagement with patients at every step of the policy-making process should be the standard we strive for.
Challenge the status quo and use your voice as a driving force for change.
Whether you want to focus on access to treatment, quality of life initiatives or research funding, you can advocate for change on behalf of Australians affected by bowel cancer.
Bowel cancer is a leading cause of death for Australians aged 25-84. Help us change that.
Consultation survey closed 19 April 21
National Preventative Health Strategy
In June 2019, the Minister for Health, the Hon Greg Hunt MP announced that the Australian Government would develop a 10-year National Preventive Health Strategy.
As a member of the Australian Patient Advocacy Alliance (APAA), Bowel Cancer Australia shares the vision of a society that prioritises prevention to reduce the current and potential impact of chronic conditions with the voices of consumers at the core of policy development; and in the APAA’s key values in the formation of a National Preventative Health Strategy.
We must improve health literacy to empower consumers and enhance their ability to navigate the health system.
We must empower and enable general practitioners to improve early diagnosis of conditions and better manage chronic conditions.
We must increase the number of specialised support nurses and consumers access to these services.
We must no longer determine the need for screening through age specific risk factors and instead pursue a model where we screen individuals according to their individual levels of risk for chronic disease.
We must evaluate the success and effectiveness of screening programs, while optimising and investing in innovation to improve chronic disease screening technologies.
We must address poor nutrition and obesity as significant indicators of, and risk factors for, developing chronic conditions to lower the burden of chronic disease.
We must empower those living with chronic conditions to incorporate exercise into their day-to-day management of their condition.
We must look beyond the physical contributions leading to obesity and address wider societal factors impacting obesity.
Consultation closed on 20 June 2022
The NBCSP Review Report has been finalised and the Department is inviting your feedback on a short Consultation Paper.
National Bowel Cancer Screening Program (NBCSP) Review
Bowel Cancer Australia advocates for expanding the NBCSP to include people aged 40-84 (currently 50-74).
Australia’s favourable overall trends in bowel cancer incidence and mortality are masking an increase in young-onset bowel cancer, an upturn that began in the mid-1990s.
Research shows 75% of all young-onset cases are diagnosed between ages 40-49.
Furthermore, a recent study demonstrated a steep increase in bowel cancer incidence between ages 49 and 50, with most cases diagnosed at an invasive stage among patients 50 years old, specifically.
This suggests these cancers were developing undetected for several years prior to diagnosis at age 50.
In response to this trend, the American Cancer Society, the American College of Gastroenterology (ACG), the United States Preventive Services Task Force (USPSTF), and the US Multi-Society Task Force (MSTF) on Colorectal Cancer updated their bowel screening guidelines to recommend that people at average risk begin screening at age 45 instead of 50.
According to a 2020 study, diagnosing cancer at an earlier stage is beneficial not just from a prevention perspective, but could also be associated with more favourable cost-benefit and resource allocation balances and should be taken into account when estimating the potential effects of expanding the screening age threshold.
Bowel Cancer Australia also advocates for:
A screening program co-designed with those it aims to benefit, complete with real and consistent engagement with consumers.
A program that respects the individual’s screening preference as screening is only effective if it gets done.
Concise, compelling and consistent messages that focus on the benefits of early detection with a clear call to action.
Participants who undergo colonoscopy following a positive test result to provide feedback about their experience using a PREMs questionnaire.
An ongoing commitment to support the health system’s capacity to provide timely colonoscopy to all Category 1 patients, to ensure the NBCSP’s overarching goal of early detection is not undermined.
-
Update: Consultation Paper released
In 2020, the Department of Health commissioned Deloitte Access Economics to undertake an independent review of the National Bowel Cancer Screening Program.
A Review Report has now been finalised and the Department is inviting your feedback on its findings. The Review Report can be found here: NBCSP Review Report.
The Department has prepared a short Consultation Paper which contains the findings from the Report and a series of discussion prompts to help gather targeted feedback. Responses are not limited to the discussion prompts and additional relevant information is welcome.
Colonoscopy wait-time guarantee
Almost 99% of bowel cancer cases can be successfully treated when detected early.
However, delays in diagnosis and treatment of patients with early-stage bowel cancer can lead to stage migration, when tumours progress from being curable by surgery (or radiotherapy) with near normal life expectancy to being incurable, with very limited life expectancy.
Prior to the pandemic, wait times for those who received a NBCSP positive screen varied between 111 and 228 days depending on where participants lived. 1 in 3 participants with a positive screen were waiting more than 6 months to learn if bowel cancer was present or not.
Colonoscopy wait times have been further compounded by the suspension of elective surgery due to COVID-19.
Bowel Cancer Australia advocates for a colonoscopy wait-time and performance guarantee:
A colonoscopy within 30 days from first healthcare presentation for people experiencing symptoms suggestive of bowel cancer or a positive screen. If wait times exceed 120 days, a prognosis can worsen if cancer is present.
Transparency and public reporting of colonoscopy wait-times by all public and private healthcare facilities, released quarterly.
Adequate funding for colonoscopy services across Australia.
Collection of patient-reported experience measures within 30 days via a questionnaire from all people who undergo a colonoscopy, asking them about their pre-procedure experience (whether people understood the risks/benefits), the hospital experience (the procedure itself, issues of dignity/privacy); and post-procedure complications (bleeding/pain), with results publicly reported.
Minimum quality standards and key performance indicators (KPIs) for the delivery of colonoscopy within Australia, along with recording and public reporting of performance against the standards and KPIs.
MBS item changes and colonoscopy services
The Government established the MBS Review Taskforce to address feedback received from clinicians and the broader community that some services on the MBS did not reflect clinical best practice.
The Taskforce worked from 2015 to 2020 to review more than 5,700 items on the MBS, including 53 items relating to colonoscopy, 85 items relating to colorectal surgery, and 105 items relating to oncology.
The Taskforce’s colonoscopy recommendations became effective from November 2019, followed by chemotherapy recommendations in November 2020, with colorectal surgery recommendations scheduled for implementation in 2022.
The new Medicare item numbers for colonoscopy are the most dramatic change since they were created, and the impact of these changes was felt immediately.
While there are no restrictions for people accessing colonoscopy if they have new symptoms or a positive screening test result, many patients with a personal or family history of the disease have been affected, resulting in significant stress and anxiety.
Patients with between 1 and 4 sub-centimetre colonic tubular adenomas with low grade dysplasia will only be allowed one colonoscopy every 5 years.
Individuals with a family history, defined as a First Degree Relative (FDR) aged below 55, or 2 FDRs of any age, or one FDR and 2 Second Degree Relatives (SDR) of any age, are allowed one colonoscopy every 5 years.
From 1 November 2019, patients will be allowed only one colonoscopy per lifetime in special circumstances when a specialist is unable to access sufficient patient information and in the specialist’s opinion there is a clinical need for a colonoscopy.
Bowel Cancer Australia is extremely concerned by the increased competition for colonoscopy services among people of varying risk; rationing of colonoscopy services through MBS changes that rely on largely evidence-poor guidelines; and any impact on a specialist’s ability to use their clinical judgement as to the most appropriate care for you when considering your circumstances and preferences.
The Medicare items and services are now being reviewed on an ongoing basis by a new Medicare Benefits Schedule (MBS) Review Advisory Committee (MRAC).
It is important the Committee understands your lived-experience and how these changes have impacted you.
The long shadow of COVID-19
Measures taken by governments to mitigate the spread of the virus and limit patient traffic have severely impacted cancer services across the country.
Cancellation of elective surgery, followed by a phased reopening at reduced capacity, has impacted the entire bowel cancer care pathway, and the consequences have been particularly acute for colonoscopy services.
Early diagnosis is a key predictor of surviving bowel cancer, therefore positive test results and symptoms need to be investigated via timely colonoscopy. Studies have long established that delays in screening, diagnostic, and surveillance colonoscopies increase the risk for bowel cancer progression and mortality.
It is now more likely that patients will present with more advanced disease, require more complex treatments and experience poorer outcomes.
In 2020, there were 11% fewer diagnostic procedures and 3% fewer bowel cancer surgeries when compared to 2019.
With 73,390 fewer colonoscopies and sigmoidoscopies and 376 fewer surgeries due to impact of COVID-19 pandemic, experts are concerned early-stage bowel cancers may continue to go undiagnosed until they reach an advanced stage, when they are more difficult to treat.
Planning for post-COVID-19 colonoscopy catch-up and ongoing capacity is urgently required to avoid cancer progression and ensure bowel cancer doesn’t become the forgotten ‘C’ in the long shadow of COVID-19.
Cancer treatments are time sensitive and stage migration is a feared complication of delayed cancer treatment. Tumours can progress from being curable by surgery (or radiotherapy) with near normal life expectancy to being incurable, with very limited life expectancy.
Australian Cancer Plan
As part of the first step in the development of the Australian Cancer Plan: a 10-year plan for national action, with 2-, 5- and 10-year priorities and goals, Bowel Cancer Australia attended a Ministerial Roundtable convened by Cancer Australia at Parliament House on 22 April 2021.
The Roundtable was an opportunity to raise ambitious ideas which address inequities and priorities across the whole cancer journey and for priority population groups.
Participants agreed on the importance of the plan providing clear national leadership and direction with a focus on reducing inequities across the cancer control continuum and fostering partnerships and collaborations. It was also agreed that the plan should be:
person-centred with a focus on understanding what is important to consumers;
equity focused and to strive for equitable cancer outcomes across all population groups;
tumour agnostic with guidance provided across all cancer types and stages;
encompassing of the cancer control continuum and considering system enablers;
future-focused, transformational and ambitious;
collaborative and encouraging of system-wide and national cooperation;
data-driven with a focus on evidence-based approaches and strengthened use of data; and
strength based while building on current successes.
To enable comprehensive and inclusive engagement and consultation with stakeholders across the cancer control continuum, the plan will be developed over two years.
Young-onset bowel cancer
1 in 10 new bowel cancer cases now occur in Australians under age 50.
Australia’s favourable overall trends in bowel cancer incidence and mortality are masking an increase in young-onset bowel cancer, an increase that began in the mid-1990s.
The trend is being driven by an increase in the number of left-sided bowel cancers, and rectal cancer in particular.
Young-onset bowel cancers are often diagnosed at later stages, suggesting the increased incidence is real and not representative of a shift in age at diagnosis attributable to earlier detection.
Of those diagnosed with young-onset bowel cancer, over 86% experience symptoms.
Bowel Cancer Australia’s Never2Young Advocacy Agenda seeks to improve care experiences and health outcomes for younger people by championing:
Greater awareness among the community and health professionals of young-onset bowel cancer.
Lower screening age below 50, in response to the increasing rates of bowel cancer in younger people.
Prompt GP referral for a colonoscopy for all people regardless of age, who present with symptoms that may be consistent with bowel cancer.
Improved pathways that ensure timely triage, diagnosis and treatment for younger people.
Better understanding the challenges faced by young-onset bowel cancer patients to improve and tailor treatment, support and care.
Further research into the causes of young-onset bowel cancer, to help build a path toward a cure.
The ‘Patient Access Gap’ - access to new treatments
The Patient Access Gap refers to the time in days patients must wait between the date a medicine is authorised for use in Australia (TGA approved/ARTG listed) and the date that it is listed on the PBS and affordably available.
Between 2010 and 2017, Australians waited an average of 820 days before being able to affordably access a medicine on the PBS, following TGA approval. Some listings for cancer treatments took even longer, despite recognition that the new medicines were clinically superior to existing options.
Bowel cancer patients have experienced some of the longest waits, with one life-extending medication taking more than six years and a record eight submissions before being listed on the PBS as a subsidised treatment.
Cancer treatment is time sensitive, and many patients don’t have time to wait, which is why Bowel Cancer Australia advocates for immediate access following approval of a drug, so patients can benefit from treatments, particularly life-saving drugs, while PBAC processes and negotiations continue.
More than 40 per cent of participants who responded to Bowel Cancer Australia’s My Cancer, My Voice survey said they would travel overseas to access a new treatment if it wasn’t available in Australia, and a similar number indicated they would write to the Government seeking local access. Will you?
Submissions closed on 17 June 2021
The report, The New Frontier - Delivering better health for all Australians, was tabled in the House of Representatives on 25 November 2021.
Inquiry into new drugs and novel medical technologies
On 18 August 2020, the House of Representatives Standing Committee on Health, Aged Care and Sport established an inquiry into the approval processes for new drugs and novel medical technologies in Australia, with a particular focus on the treatment of rare diseases and conditions where there is a high and unmet clinical need. With just over one in ten (13.4%) mCRC patients surviving five years after diagnosis, there is a clear clinical need for new treatment options.
The Inquiry reviewed issues preventing Australians from accessing options that are often a standard of care globally but are currently unavailable in Australia.
In our written submission and in-person testimony, Bowel Cancer Australia highlighted one such life-extending medication which took more than six years and a record eight submission before it was listed on the PBS as a subsidised treatment for bowel cancer patients.
To improve access to new drugs and novel medical technologies, Bowel Cancer Australia is advocating for:
a return to the process of automatic listing on the PBS for bowel cancer treatments that have received a positive PBAC recommendation.
an agile approval system to facilitate timely access to treatments and technologies.
immediate access for patients following a positive PBAC recommendation so patients can benefit from new treatments whilst the PBAC process and negotiations continue.
In addition, Bowel Cancer Australia joined with members of the Australian Patient Advocacy Alliance in a separate submission, urging the committee to explore the following recommendations:
Develop a health technology process that puts patients at its core.
Optimise the funding envelope with streamlined governance and a focus on outcomes.
Improve HTA process and timeliness to bring treatments more rapidly within reach of all Australian patients.
Further strengthen the transparency of the process that makes new therapies available.
-
Update: Committee releases report on reforming the process for new medicines and health technology
The report, The New Frontier - Delivering better health for all Australians, was tabled in the House of Representatives on 25 November 2021.
Throughout the 15-month inquiry, the Committee received over 200 submissions and held 13 days of public hearings.
The report makes 31 recommendations to reform Australia’s system for the regulation and reimbursement with the hope that patients will receive faster access to the latest medicines and technologies.
National Medicines Policy (NMP) Review
Since 1999, the NMP has served as the guiding principle underpinning all policy decisions related to access and reimbursement of medicines, safety and quality of medicines, medicine management, and industry policy.
While these principles remain admirable and fundamental, there is broad consensus among APAA members that the NMP no longer remains fit for purpose.
The APAA brings together peak health advocacy organisations representing more than 12 million people living with chronic and complex health conditions, with the purpose of providing a coordinated and cohesive approach to government.
As a member of APAA, Bowel Cancer Australia welcomes the review of the NMP and shares in the Alliance’s vision of a National Medicines Policy that supports all Australians to receive the therapeutics they need at the right time to deliver better health outcomes; a system that enhances consumer participation; is flexible and adapts to advances in technology; and is transparent with established measures to evaluate the success of how it is delivered.
Once therapeutics are funded, decisions on what is available are too often restricted based on the state/territory where patients live, down to the local Primary Health Networks patients are seen in, which leads to considerable disparities.
A review must work with state and territory governments to strengthen the availability of therapies once a drug is funded and ensure equity of access is guaranteed for all Australians no matter where they live.
Additionally, the NMP must consider the economic burden on patients, which too often is a barrier to patient access, particularly for those living with chronic or complex conditions, often with co-morbidities requiring multiple treatments.
While we acknowledge access to therapeutics must come at a cost the community can afford, we believe there is an opportunity to update the determinants of medicine effectiveness and cost-effectiveness to better reflect environmental and market change to improve the equity of treatment for all patients.
Together, we seek a National Medicines Policy that will:
Ensure Australians can access the therapeutics they need no matter how much they earn or where they live. The NMP must provide a framework that enables program delivery to address the inherent and systematic issues leading to inequity and lack of access.
Broaden the definition to ensure advanced therapies such as new treatments, diagnostics and medical devices are recognised as part of the therapeutics landscape.
Provide a framework that is fit for purpose and enables processes that are flexible to adapt to advances in constantly evolving technology to ensure timely access for all.
Place patients at the core with a policy that empowers informed patient participation, closing the gap between patients and decisions.
Prioritise effective and clear communication to enable increased transparency in the process leading to improved engagement by consumers. Additionally, evaluation and performance measures must be established in the NMP to ensure it is working where it matters.
Embed end-to-end transparency as a core principle to ensure trust in the system that supports access to the medicines Australians need.
Health Technology Assessment (HTA) Review
The Federal Government has agreed to support an independent review of the current Health Technology Assessment (HTA) policy and the methods used by the Pharmaceutical Benefits Advisory Committee (PBAC) to assess new medicines for listing on the Pharmaceutical Benefits Scheme (PBS). The review will explore contemporary research and relevant methodologies and purchasing practices used by comparable international jurisdictions as part of the process.
The review will be overseen by a committee that is independently chaired, and includes a patient representative, a member nominated by Medicines Australia, a Government Nominee, the Chair of the PBAC and the independent Chair.
The review will be conducted during the 2022-23 financial year and will help ensure Australia is a global priority for launching new medicines and delays in accessing innovative therapies for patients are reduced.
Bowel Cancer Australia is calling for the recommendations of this review to lead to improvements in the process enabling better access to treatments, improved equity of care for all Australians, and a mechanism to regain the trust of all involved.
We expect the HTA review to work alongside the National Medicines Policy review and other initiatives, in seeking improvements to ensure the system is flexible and agile. This will create a system that can respond to technological advancements, standards, and sources of evidence necessary to support funding applications including global, and real-world data, and assess the basis and consideration of risk that is currently used.
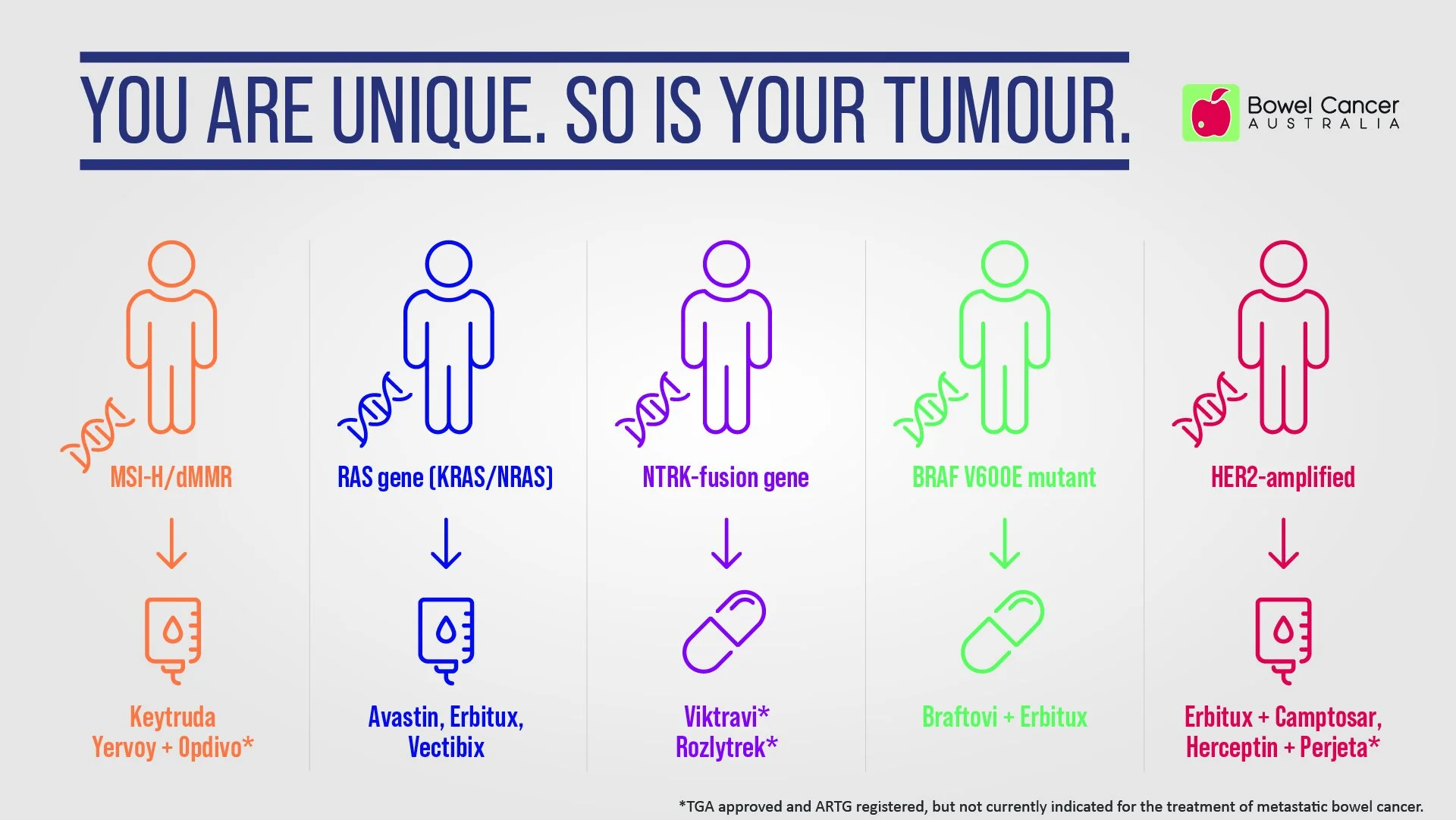
Biomarker testing - a powerful tool in treatment planning
You are unique. So is your tumour.
That means your bowel tumour might not respond to the same treatment that another bowel cancer patient receives.
Biomarkers, short for biological markers or molecular markers, have characteristics that enable them to be measured and evaluated as an indicator of normal processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.
Biomarkers include DNA, proteins, and genetic mutations found in blood, tissue, or other body fluids. Your ‘biomarker profile’ can help you and your doctor personalise your treatment.
Biomarkers for bowel cancer are used for diagnosis, progression, prognosis, and for treatment. Some biomarkers have both a prognostic and a predictive value.
Diagnostic biomarkers detect the disease.
Prognostic biomarkers are associated with a clinical outcome regardless of the treatment received.
Predictive biomarkers can predict the benefit or lack of benefit of a treatment.
Bowel Cancer Australia advocates for:
Biomarker testing as soon as possible after diagnosis and before a treatment plan is agreed.
Equity in access to new biomarker tests for targeted therapies and the expertise to apply the results in clinical decision making.
Improved processes for developing and updating clinical practice guidelines for the effective use of biomarker tests for targeted therapies.
Regardless of stage, Bowel Cancer Australia advocates for biomarker testing all bowel cancers for mismatch repair deficiency to subsequently identify Lynch syndrome, a dominant hereditary condition caused by mutations in MMR genes. Around 3-5% of all bowel cancers can be attributed to Lynch syndrome.
Testing for biomarkers such as microsatellite instability, RAS mutations, and BRAF V600E helps you and your treatment team develop a personalised treatment plan just for you and your biomarker status can help determine if you’re eligible for any clinical trials.
New knowledge and improved testing has shown that additional mutations including HER2 and neurotrophic tyrosine receptor kinase (NTRK), may also play a role in resistance to certain treatments and responsiveness to others in metastatic bowel cancer patients.
Stomal Therapy Nurses
There are currently estimated to be 44,000 Australians living with a stoma.
Stomal therapy nurses play an important role in caring for patients living with a temporary or permanent stoma, often as a result of bowel surgery for cancer or inflammatory bowel diseases.
They can provide pre- and post-operative support and advice on what to expect if this operation is being considered, including optimal placement of the stoma. They can also assist with questions regarding stoma care and management, and stoma reversal.
Stomal therapy nurses have undertaken specialist education and training to help patients manage physical, social and emotional adjustments as well as provide advice and practical support to help overcome fear of living with ‘a bag’, which is common for bowel cancer patients.
Stomal therapy nurses assist patients to access stomal therapy products available under the Australian Government Stoma Appliance Scheme.
According to the MBS Review Taskforce Report from the Colorectal Surgical Clinical Committee, there is wide disparity in access to services provided by stomal therapy nurses across Australia.
It is often more difficult for private hospital patients and for patients in regional and remote areas to access stomal therapy services, particularly following discharge from hospital.
Access to services is further impaired by a shortage of nurses with sufficient stomal therapy qualifications. This may compromise appropriate patient care as well as impact on physical, social and emotional patient outcomes.
At present, there is no provision within the MBS for stomal therapy services.
Bowel Care Nurses
Results from Bowel Cancer Australia’s national patient survey, My Cancer, My Voice, revealed bowel cancer patients at all stages of the disease felt they had the 'wrong cancer', due to a lack of dedicated support services and low awareness of the disease.
Although bowel cancer is the third most diagnosed cancer, bowel cancer patients don’t receive the same level of support as other common cancers.
One-third of bowel cancer patients reported seeing between four and six health professionals and in 16% of cases, patients reported having to see more than seven health professionals when coordinating their care.
To date, the Federal Government has committed around $120 million for 98 dedicated breast cancer nurse specialists, $33 million for 62 prostate nurse specialists, and $6.9 million for 5 lung cancer nurse specialists.
However, Federal Government nurse funding has never been committed to better support bowel cancer patients during their treatment and care. It’s little wonder why bowel cancer patients report feeling they have been diagnosed with the ‘wrong cancer’ when it comes to receiving care and support.
A Bowel Care Nurse is a registered nurse who has specialist knowledge and experience caring for patients with bowel cancer, and every bowel cancer patient deserves access to one.
Access to a nurse specialist by phone or in-person was identified by more than 8 in 10 (83%) patients as an important resource to improve their care coordination.
To address this need, Bowel Cancer Australia has been offering telenursing and telenutritionist support for over a decade. Described as a 'lifeline' by patients and their loved ones, the service enables access to personalised care and tailored support nationwide.
More recently we have funded in-person nurses in regional communities who act as a point of contact for bowel cancer patients and their families as they navigate the health system.
Bowel Cancer Australia remains committed to growing the number of specialised bowel care nurses to improve patient experiences and outcomes, supported by a sustainable funding model.
Patient-reported measures
Medical guidelines are not tailored to patient circumstances or preferences and standardised care pathways do not guarantee standardised outcomes, as neither measure patient-reported outcomes - the only metric that directly captures what a patient cares about most and whether health procedures they undertake actually make them feel better.
That’s why Bowel Cancer Australia, in a world-first, co-sponsored development of the Colorectal Cancer Standard Set in collaboration with the International Consortium for Health Outcomes Measurement (ICHOM), health professionals, researchers and most importantly, patients.
Soon after, we launched My Colonoscopy Experience and My Bowel Cancer, My Voice online questionnaires so patients can capture and share their personal perspectives about their health, healthcare experiences and the impact bowel cancer and treatment are having on their wellbeing.
Bowel Cancer Australia has joined the Australian Patient Advocacy Alliance in calls for the Federal Government to fund a feasibility study to consider the establishment of a national system to capture patient-reported experience measures (PREMs) and patient-reported outcome measures (PROMs) to drive better clinical practice and patient outcomes.
The design of the system should be patient-led (consumer-led) and guided by the work of the International Consortium for Health Outcomes Measurement (ICHOM).
While Bowel Cancer Australia will continue to advocate for PREMs and PROMs, you can participate in our My Colonoscopy Experience and My Bowel Cancer, My Voice online questionnaires today.
Cancer Survivorship Care
Together with 30 other community support organisations, Bowel Cancer Australia has endorsed a Cancer Survivorship Care Consensus Statement to articulate the vital role our organisations play in the provision of survivorship care to Australians of all ages who have been diagnosed with cancer.
Collectively, community support organisations:
Are a critical third sector of the Australian health system, targeting a multitude of health conditions, delivering essential services, support, resources and research contributions that relieve considerable burden on the primary and acute care sectors.
Address many of the care-navigation, psychosocial and information needs of cancer survivors and those affected by cancer.
Have flexible offerings allowing a person-centred approach to service delivery that is responsive to individual needs.
Have a focus on health advocacy, through work with government and health service policy makers to ensure equitable and affordable access to best practice survivorship care.
Take a substantial leadership role in development and delivery of health promotion interventions to address cancer risk behaviours.
Address equitable access issues by typically providing their services and support at low or no cost to service users, often in non-hospital settings close to where cancer survivors live.
Making bowel cancer research a priority
Bowel cancer is Australia's second deadliest cancer and has the second highest disease burden of any cancer in Australia. Burden of disease measures the impact of living with illness and injury or dying prematurely.
However, bowel cancer received around 55 percent less research funding from the National Health and Medical Research Council (NHMRC) compared to breast cancer (2021: $8.6 million v $19.1 million).
To help address this research funding inequity, Bowel Cancer Australia established the Bowel Cancer Research Foundation Australia.
An objective of the Foundation is to support and sustainably fund research into the causes, prevention and treatment of bowel cancer to benefit us all in the future.
Bowel Cancer Australia has committed $12.54 million to bowel cancer research projects in collaboration with funding partners, including a $10.4 million investment in the Lawrence Penn Chair of Bowel Cancer Research and mass spectrometry core facility, dedicated to leading edge bowel cancer discoveries.
The establishment of the Lawrence Penn Chair, in collaboration with the University of Sydney and the Kolling Institute, strengthens our collective pursuit of a bowel cancer cure.
Increased research funding equals better treatment options and ultimately, discovery of a cure.